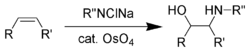
Enantioselective synthesis
As a result, living systems possess a high degree of chemical chirality and will often react differently with the various enantiomers of a given compound.
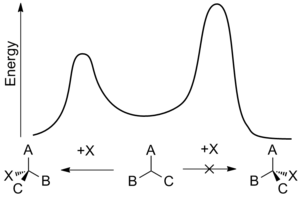
Enantiomers possess identical enthalpies and entropies and hence should be produced in equal amounts by an undirected process – leading to a racemic mixture.
For example, Noyori asymmetric hydrogenation with BINAP/Ru requires a β-ketone, although another catalyst, BINAP/diamine-Ru, widens the scope to α,β-alkenes and aromatic chemicals.
However, in some cases the only available stereoselective methodology relies on chiral auxiliaries and these reactions tend to be versatile and very well-studied, allowing the most time-efficient access to enantiomerically pure products.
[18] Additionally, the products of auxiliary-directed reactions are diastereomers, which enables their facile separation by methods such as column chromatography or crystallization.
Biocatalysis makes use of biological compounds, ranging from isolated enzymes to living cells, to perform chemical transformations.
Organocatalysis refers to a form of catalysis, where the rate of a chemical reaction is increased by an organic compound consisting of carbon, hydrogen, sulfur and other non-metal elements.
[28] Organocatalysis often employs natural compounds and secondary amines as chiral catalysts;[29] these are inexpensive and environmentally friendly, as no metals are involved.
A readily available chiral starting material is manipulated through successive reactions, often using achiral reagents, to obtain the desired target molecule.
However, the number of possible reactions the molecule can undergo is restricted and tortuous synthetic routes may be required (e.g. Oseltamivir total synthesis).
In 1815 the French physicist Jean-Baptiste Biot showed that certain chemicals could rotate the plane of a beam of polarised light, a property called optical activity.
[32] The nature of this property remained a mystery until 1848, when Louis Pasteur proposed that it had a molecular basis originating from some form of dissymmetry,[33][34] with the term chirality being coined by Lord Kelvin a year later.
[35] The origin of chirality itself was finally described in 1874, when Jacobus Henricus van 't Hoff and Joseph Le Bel independently proposed the tetrahedral geometry of carbon.
In 1894 Hermann Emil Fischer outlined the concept of asymmetric induction;[39] in which he correctly ascribed selective the formation of D-glucose by plants to be due to the influence of optically active substances within chlorophyll.
[43] Unlike Fischer, Marckwald had performed an enantioselective reaction upon an achiral, un-natural starting material, albeit with a chiral organocatalyst (as we now understand this chemistry).
[38][44][45] The development of enantioselective synthesis was initially slow, largely due to the limited range of techniques available for their separation and analysis.
First synthesized in 1953, thalidomide was widely prescribed for morning sickness from 1957 to 1962, but was soon found to be seriously teratogenic,[51] eventually causing birth defects in more than 10,000 babies.
The disaster prompted many countries to introduce tougher rules for the testing and licensing of drugs, such as the Kefauver-Harris Amendment (US) and Directive 65/65/EEC1 (EU).
[53][54] The same year saw first successful enantiomeric separation by gas chromatography[55] an important development as the technology was in common use at the time.
Metal-catalysed enantioselective synthesis was pioneered by William S. Knowles, Ryōji Noyori and K. Barry Sharpless; for which they would receive the 2001 Nobel Prize in Chemistry.
Enzyme-catalyzed enantioselective reactions became more and more common during the 1980s,[66] particularly in industry,[67] with their applications including asymmetric ester hydrolysis with pig-liver esterase.
The emerging technology of genetic engineering has allowed the tailoring of enzymes to specific processes, permitting an increased range of selective transformations.
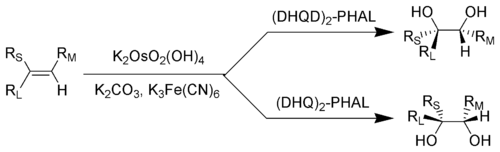
Key: R L = Largest substituent; R M = Medium-sized substituent; R S = Smallest substituent
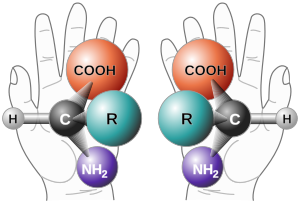





Left: ( S )-thalidomide
Right: ( R )-thalidomide