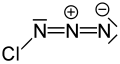Chlorine azide
Chlorine azide (ClN3) is an inorganic compound that was discovered in 1908 by Friedrich Raschig.
[2] Concentrated ClN3 is notoriously unstable and may spontaneously detonate at any temperature.
It may explode, sometimes even without apparent provocation; it is thus too sensitive to be used commercially unless first diluted in solution.
On contact with acid, chlorine azide decomposes, evolving toxic and corrosive hydrogen chloride gas.
[5] Its shipment is subject to strict reporting requirements and regulations by the US Department of Transportation.