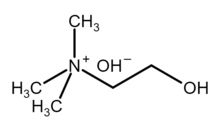
Choline hydroxide
It is hygroscopic and thus often encountered as a colorless viscous hydrated syrup that smells of trimethylamine (TMA).
The cation of this salt, choline, occurs in nature in living beings.
[8] Choline hydroxide is used in industry as a pH regulating agent[1] and as an eco-friendly, biodegradable, recyclable and efficient catalyst with high yields for synthesis of certain organic compounds (2-amino-3-nitro-4H-chromene derivatives) in an aqueous solution at room temperatures.
[2] Choline hydroxide irritates skin, eyes and respiratory system.
Inhalation of this chemical may cause dyspnea and corrosive injuries to upper respiratory system and lungs, which can lead to pneumonia.