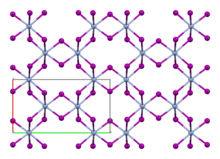
Chromium(III) iodide
In this structure, chromium exhibits octahedral coordination geometry.
The reaction is conducted at 500 °C: To obtain high purity samples, the product is thermally decomposed at 700 °C to sublime out chromium(II) iodide.
Like CrCl3, the triiodide exhibits slow solubility in water owing to the kinetic inertness of Cr(III).
Addition of small amounts of chromous iodide accelerates the dissolving process.
[2] Chromium triiodide can also be prepared as nanoplatelets[clarification needed] from the alkoxide Cr(OC(CH3)(C(CH3)3)2)3.[4][how?]