PA clan of proteases
[1][2] PA clan proteases can be found in plants,[3] animals,[3] fungi,[3] eubacteria,[4] archaea[5][6] and viruses.
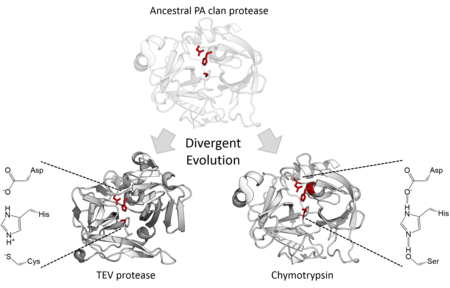
[7] The differences in the catalytic triad within the PA clan is also an example of divergent evolution of active sites in enzymes.
[2][10][11] Based on structural homology, a superfamily was defined and later named the PA clan (by the MEROPS classification system).
Members of the PA clan can be found in eukaryotes, prokaryotes and viruses and encompass a wide range of functions.
Several snake venoms are also PA clan proteases, such as pit viper haemotoxin and interfere with the victim's blood clotting cascade.
Additionally, bacteria such as Staphylococcus aureus secrete exfoliative toxin which digest and damage the host's tissues.
[19][20] There are also several pseudoenzymes in the superfamily, where the catalytic triad residues have been mutated and so function as binding proteins.