Clinical neurochemistry
Clinical neurochemistry is related to neurogenesis, neuromodulation, neuroplasticity, neuroendocrinology, and neuroimmunology in the context of associating neurological findings at both lower and higher level organismal functions.
This understanding of drug action in turn can be extrapolated to account for system-wide or clinical manifestations which are observed as symptoms.
Because many receptors are essentially enzymes, the field of pharmakinetics utilizes the Michaelis–Menten equation to describe drug affinity (dissociation constant Kd) and total binding (Bmax).
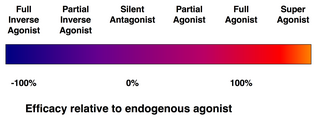
[2] Naloxone, an antagonist of the opioid receptors, exerts a biological effect only be interfering with endogenous neurotransmitter (morphine) binding.
Peripheral nociceptors uniquely express transient receptor potentials which are sensitive to potentially-damaging mechanical, chemical, or thermal stimuli.
[3] The dorsal horn of the spinal cord serves as a major integration center for both ascending nociceptive information and descending antinociceptive influences from the brain.
Plasticity within the dorsal horn is mediated by NMDA glutamate receptors and key in the initiation of chronic pain by decreasing the excitability threshold in nociceptive pathways.
[4] Three families in northern Pakistan were congenitally unable to perceive pain due to their homozygous loss of function mutation in the SCN9A gene which codes for the voltage-gated sodium channel NaV1.7 .
This key finding allowed pharmacologists to begin researching if the NaV1.7 is a substantial molecular target for analgesic (antipain) medications.
[5] Sensitization, in the clinical sense of the word, is a phenomenon in which nociceptors in an area beyond a tissue injury exhibit decreased thresholds for activation.
Sensitization can be initiated by inflammatory prostaglandins or leukotrienes and are therefore the targets of nonsteroidal anti-inflammatory (NSAIDs) which block key enzymes in their synthesis.
Circadian rhythms are produced in the suprachiasmatic nucleus by pacemaker cells which contain transcriptional regulation "clock genes" which have been highly conserved throughout evolution.
The mechanism of narcolepsy is unknown, though recent findings suggest that orexin neurons in the lateral and posterior hypothalamus may play a critical role in reinforcing wakefulness.
Schizophrenia is a psychological disorder in which a patient experiences symptoms including hallucinations, delusions, amotivation, social withdrawal, cognitive defects, and poor working memory.
[8] Schizophrenia is anatomically characterized by a deterioration and loss of gray matter in the temporal and frontal regions of the cerebral cortex, though the exact mechanism is unknown.
[10] Increased levels of dopamine in people with schizophrenia tend to induce paranoid delusions, ideas of reference, and auditory hallucinations.
Surprisingly, both necrotic and apoptotic processes utilize a similar intracellular signaling cascade which uses caspase proteins to induce cell death.
However, these attempts were unsuccessful and the only clinically useful drugs used in the United States are cholinesterase inhibitors, which prolong the time before choline degradation.
Parkinson's disease is caused by the loss of dopamine stimulation to the basal ganglia of the midbrain, resulting in tremor at rest and bradykinesia.
Deep brain stimulation offsets symptoms rather than cures, and stem cell studies have been extremely disappointing, despite relative success with animal models.
Via an unknown mechanism, this accumulation leads to neurodegeneration in the caudate nucleus and putamen, selectively destroying GABAergic neurons which project to the globus pallidus.
Treatment for Huntington's disease is extremely limited due to the lack of knowledge concerning the pathogenesis of protein accumulation, though drugs used include dopamine receptor antagonists to minimize tremors and antidepressants to ameliorate symptoms of psychosis and depression.