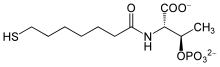
Coenzyme B
[1] The molecule contains a thiol, which is its principal site of reaction.
Coenzyme B reacts with 2-methylthioethanesulfonate (methyl-Coenzyme M, abbreviated CH3–S–CoM), to release methane in methanogenesis:[2] This conversion is catalyzed by the enzyme methyl coenzyme M reductase, which contains cofactor F430 as the prosthetic group.
A related conversion that utilizes both HS-CoB and HS-CoM is the reduction of fumarate to succinate, catalyzed by fumarate reductase:[3] Coenzyme B is an important component in the terminal step of methane biogenesis.
[4] It acts as a two electron-donor to reduce coenzyme M (methyl-coenzyme) into two molecules a methane and a heterodisulfide.
[5] Two separate experiments that were performed, one with coenzyme B and other without coenzyme B, indicated that using coenzyme B before the formation of the methane molecule, results in a more efficient and consistent bond cleavage.