Coenzyme A
[2] In its acetyl form, coenzyme A is a highly versatile molecule, serving metabolic functions in both the anabolic and catabolic pathways.
Its structure was determined during the early 1950s at the Lister Institute, London, together by Lipmann and other workers at Harvard Medical School and Massachusetts General Hospital.
He was able to isolate and purify the factor from pig liver and discovered that its function was related to a coenzyme that was active in choline acetylation.
[6] Work with Beverly Guirard, Nathan Kaplan, and others determined that pantothenic acid was a central component of coenzyme A.
In 1953, Fritz Lipmann won the Nobel Prize in Physiology or Medicine "for his discovery of co-enzyme A and its importance for intermediary metabolism".
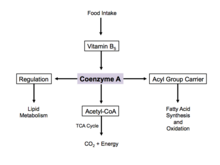
[6][9] Coenzyme A is naturally synthesized from pantothenate (vitamin B5), which is found in food such as meat, vegetables, cereal grains, legumes, eggs, and milk.
[16] A 2024 article[citation needed] detailed a plausible chemical synthesis mechanism for the pantetheine component (the main functional part) of coenzyme A in a primordial prebiotic world.
Coenzyme A is produced commercially via extraction from yeast, however this is an inefficient process (yields approximately 25 mg/kg) resulting in an expensive product.
[17] Since coenzyme A is, in chemical terms, a thiol, it can react with carboxylic acids to form thioesters, thus functioning as an acyl group carrier.
This process is the body's primary catabolic pathway and is essential in breaking down the building blocks of the cell such as carbohydrates, amino acids, and lipids.
Mammalian and bacterial cells subjected to oxidative and metabolic stress show significant increase in the covalent modification of protein cysteine residues by coenzyme A.
Using anti-coenzyme A antibody[25] and liquid chromatography tandem mass spectrometry (LC-MS/MS) methodologies, more than 2,000 CoAlated proteins were identified from stressed mammalian and bacterial cells.