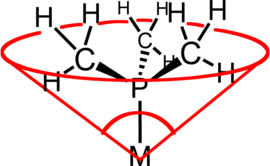
Ligand cone angle
Tolman originally developed the method for phosphine ligands in nickel complexes, determining them from measurements of accurate physical models.
The metal-ligand bond length in the Tolman model was determined empirically from crystal structures of tetrahedral nickel complexes.
In contrast, the solid-angle concept derives both bond length and the perimeter from empirical solid state crystal structures.
The concept of cone angle is of practical importance in homogeneous catalysis because the size of the ligand affects the reactivity of the attached metal center.
Recent research has found that other descriptors—such as percent buried volume—are more accurate than cone angle at capturing the relevant steric effects of the phosphine ligand(s) when bound to the metal center.