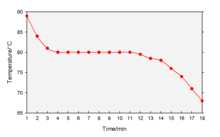
Cooling curve
When the phase change occurs, there is a "thermal arrest"; that is, the temperature stays constant.
This is because the matter has more internal energy as a liquid or gas than in the state that it is cooling to.
The amount of energy required for a phase change is known as latent heat.
First, the molten alloy reaches to liquidus temperature and then freezing range starts.
You can help Wikipedia by expanding it.This article about statistical mechanics is a stub.