Superheating
[1][2] Superheating is achieved by heating a homogeneous substance in a clean container, free of nucleation sites, while taking care not to disturb the liquid.
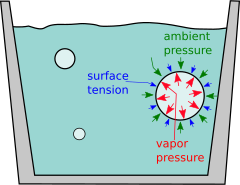
[4] Surface tension makes the bubble act like an elastic balloon.
What makes superheating so explosive is that a larger bubble is easier to inflate than a small one; just as when blowing up a balloon, the hardest part is getting started.
This means if the largest bubbles in a container are small, only a few micrometres in diameter, overcoming the surface tension may require a large
Once a bubble does begin to grow, the surface tension pressure decreases, so it expands explosively in a positive feedback loop.
In practice, most containers have scratches or other imperfections which trap pockets of air that provide starting bubbles, and impure water containing small particles can also trap air pockets.
Superheating can occur when an undisturbed container of water is heated in a microwave oven.
At the time the container is removed, the lack of nucleation sites prevents boiling, leaving the surface calm.
[6] The boiling can be triggered by jostling the cup, inserting a stirring device, or adding a substance like instant coffee or sugar.
The chance of superheating is greater with smooth containers, because scratches or chips can house small pockets of air, which serve as nucleation points.
Superheating is more likely after repeated heating and cooling cycles of an undisturbed container, as when a forgotten coffee cup is re-heated without being removed from a microwave oven.
This is due to heating cycles releasing dissolved gases such as oxygen and nitrogen from the solvent.
To avoid a dangerous sudden boiling, it is recommended not to microwave water for an excessive amount of time.