Copper(I) sulfide
[5] Cu2S reacts with oxygen to form SO2:[6] The production of copper from chalcocite is a typical process in extracting the metal from ores.
Usually, the conversion involves roasting, to give Cu2O and sulfur dioxide:[6] Cuprous oxide readily converts to copper metal upon heating.
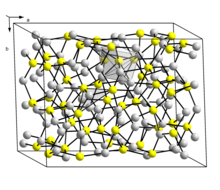
The so-called low temperature monoclinic form ("low-chalcocite") has a complex structure with 96 copper atoms in the unit cell.
With the approximate formula Cu1.96S, this material is non-stoichiometric (range Cu1.934S-Cu1.965S) and has a monoclinic structure with 248 copper and 128 sulfur atoms in the unit cell.
[9] The electrical resistivity increases abruptly at the phase transition point around 104 °C, with the precise temperature depending on the stoichiometry.