Corannulene
Flash vacuum pyrolysis techniques generally have lower chemical yields than solution-chemistry syntheses, but offer routes to more derivatives.
[6] One synthesis based on solution chemistry[7] consists of a nucleophilic displacement–elimination reaction of an octabromide with sodium hydroxide: The bromine substituents are removed with an excess of n-butyllithium.
Penta-substituted fullerenes (with methyl or phenyl groups) charged with five electrons form supramolecular dimers with a complementary corannulene tetraanion bowl, 'stitched' by interstitial lithium cations.
Concave binding has been reported for a cesium / crown ether system [24] UV 193-nm photoionization effectively removes a π-electron from the twofold degenerate E1-HOMO located in the aromatic network of electrons yielding a corannulene radical cation.
[25] Owing to the degeneracy in the HOMO orbital, the corannulene radical cation is unstable in its original C5v molecular arrangement, and therefore, subject to Jahn-Teller (JT) vibronic distortion.
The molecule's stereochemistry consists of two chiral elements: the asymmetry of a singly substituted corannulenyl, and the helical twist about the central bond.
In the neutral state, bicorannulenyl exists as 12 conformers, which interconvert through multiple bowl-inversions and bond-rotations.
[27] When bicorannulenyl is reduced to a dianion with potassium metal, the central bond assumes significant double-bond character.
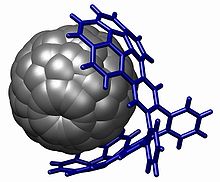
The corannulene group is used in host–guest chemistry with interactions based on pi stacking, notably with fullerenes (the buckycatcher) [30][31] but also with nitrobenzene[32] Alkyl-substituted corannulenes form a thermotropic hexagonal columnar liquid crystalline mesophase.


![Cyclopenta[bc]corannulene](http://upload.wikimedia.org/wikipedia/commons/thumb/2/21/Cyclopenta-bc-corannulene.png/100px-Cyclopenta-bc-corannulene.png)