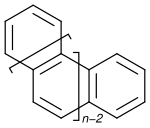
Helicene
In organic chemistry, helicenes are ortho-condensed polycyclic aromatic compounds in which benzene rings or other aromatics are angularly annulated to give helically-shaped chiral molecules.
[1] The chemistry of helicenes has attracted continuing attention because of their unique structural, spectral, and optical features.
The stability of the two complementary helical enantiomers with respect to interconversion and the mechanism by which they interconvert depend on n.[9] The first helicene structure was reported by Jakob Meisenheimer in 1903 as the reduction product of 2-nitronaphthalene.
[11] The first [6]helicene (also called hexahelicene) was synthesized by M. S. Newman and D. Lednicer in 1955 via a scheme that closed the two central rings by Friedel–Crafts cyclization of carboxylic acid compounds.
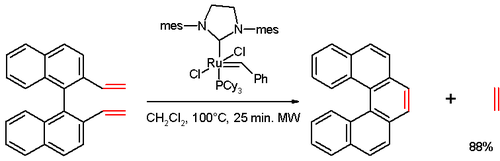
[14] In one study,[15] [5]helicene was synthesized in an olefin metathesis reaction of a divinyl compound (prepared from 1,1′-bi-2-naphthol (BINOL) in several steps), with Grubbs' second generation catalyst: Other approach is also non-photochemical and is based on assembly of biphenylyl-naphthalenes and their platinum-catalyzed double cycloisomerization leading to various [6]helicenes:[16] Helicenes have been studied with respect to nonlinear optics,[17] CPL,[18][19] organocatalysis,[20] conformational analysis,[21] chirality sensing,[22] chemical sensors[23] and hetero-atom substitution.