Zethrene
Zethrene (dibenzo[de,mn]naphthacene) is a polycyclic aromatic hydrocarbon consisting of two phenalene units fused together.
Zethrene has a deep-red color and it is light sensitive - complete decomposition under a sunlight lamp occurs within 12 hours.
The compound was originally synthesized by Erich Clar in 1955[1] from acenaphthene in one method and from chrysene in another.
[2][3] A sulfur extrusion method was reported by Kemp, Storie, and Tulloch.
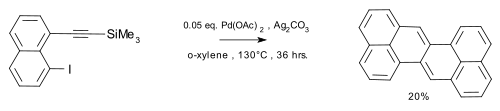
[4] Wu et al.[5] reported the synthesis of the compound in a coupling reaction / dimerization with in-situ desilylation.