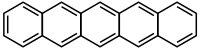
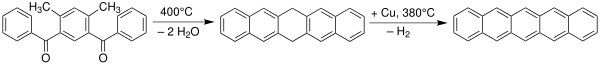
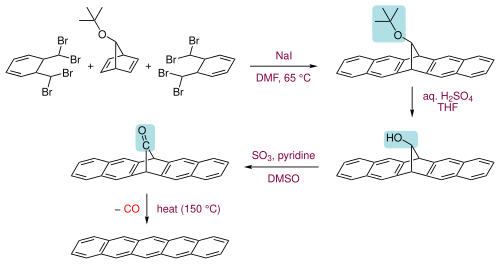
Pentacene
In August 2009, a group of researchers from IBM published experimental results of imaging a single molecule of pentacene using an atomic force microscope.
[1][2] In July 2011, they used a modification of scanning tunneling microscopy to experimentally determine the shapes of the highest occupied and lowest unoccupied molecular orbitals.
[5] The compound, originally called dinaphthanthracene after naphthalene and anthracene (modern nomenclature for polyacenes, including pentacene, was only introduced in 1939 by Erich Clar[7][8]), was first synthesized in 1912 by British chemists William Hobson Mills and Mildred May Gostling.
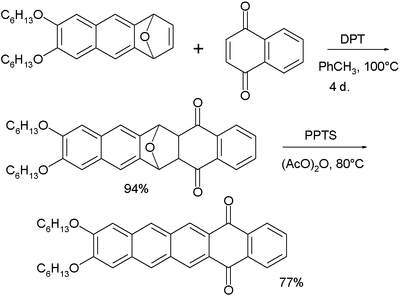
6,13-Substituted pentacenes are accessible through pentacenequinone by reaction with an aryl or alkynyl nucleophile (for example Grignard or organolithium reagents) followed by reductive aromatization.
[14][15][16] Another method is based on homologization of diynes by transition metals (through zirconacyclopentadienes) [17][18][19][20][21] Functionalization of pentacene has allowed for control of the solid-state packing of this chromophore.
According to one study[24] the reaction mechanism for this equilibrium is not based on an intramolecular 1,5-hydride shift, but on a bimolecular free radical hydrogen migration.
The compound can be resolved into its two enantiomers with an unusually high reported optical rotation of 7400° although racemization takes place with a chemical half-life of 9 hours.
[35] A modular synthetic method to conjugated pentacene di-, tri- and tetramers (6–8) has been reported which is based on homo- and cross-coupling reactions of robust dehydropentacene intermediates.
If the pentacene is preoxidized, the pentacene-quinone is a potential gate insulator, then the mobility can approach that of rubrene – the highest-mobility organic semiconductor – namely, 40 cm2/(V·s).