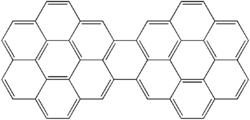
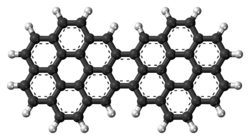
Dicoronylene
Dicoronylene does undergo a Diels–Alder reaction with maleic anhydride on one or both of the central bay regions on either side of the bridging ring.
After these were extracted and identified, a reddish residue remained, which was sparingly soluble in organic solvents.
Dicoronylene was later discovered to occur as a by-product of the catalytic hydrocracking used in petroleum processing.
It is estimated that catalytical hydrocracking produces several hundred metric tons of dicoronylene worldwide per year, making it the most prevalent large PAH.
This causes plugging of flow lines that require periodic shutdown and removal of the reddish deposits.