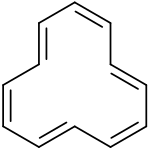
Cyclododecahexaene
[citation needed] On the other hand the dianion with 14 electrons is a Hückel aromat and more stable.
The first [12]annulene with sym-tri-trans configuration was synthesized in 1970 from a tricyclic precursor by photolysis at low temperatures.
[2] In one study the 1,7-ditrans isomer is generated at low temperatures in THF by dehydrohalogenation of a hexabromocyclododecane with potassium tert-butoxide.
Reduction of this compound at low temperature with caesium metal leads first to the radical anion and then to the dianion.
The chemical shift for the internal protons in this compound is with +0.2 ppm much more modest than in the tri-trans isomer.
![[12]annulene synthesis](http://upload.wikimedia.org/wikipedia/commons/thumb/8/84/Cyclododecahexaene_synthesis.svg/500px-Cyclododecahexaene_synthesis.svg.png)
![[12]annulene synthesis](http://upload.wikimedia.org/wikipedia/commons/thumb/d/db/12annulene2006.png/400px-12annulene2006.png)