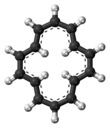
Cyclotetradecaheptaene
Cyclotetradecaheptaene, often referred to as [14]annulene, is a hydrocarbon with molecular formula C14H14, which played an important role in the development of criteria (Hückel's rule) for aromaticity, a stabilizing property of central importance in physical organic chemistry.
[1] Although the conjugated ring of [14]annulene contains 4n+2 electrons, it only exhibits limited evidence for being aromatic.
[3] Its 1H NMR spectrum shows evidence of aromatic ring currents that result in an upfield shift for the interior hydrogens.
In contrast, the corresponding [12]- and [16]annulenes, which are weakly antiaromatic or nonaromatic, have downfield shifted interior hydrogens.
However, unlike the undoubtedly aromatic [18]annulene, [14]annulene does not bear the hallmark aromatic property of chemical stability, and it quickly decomposes when exposed to light and air.