Cytochrome P450
P450s are, in general, the terminal oxidase enzymes in electron transfer chains, broadly categorized as P450-containing systems.
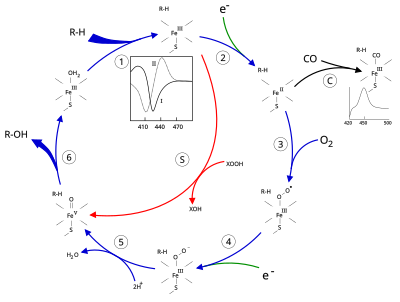
The term "P450" is derived from the spectrophotometric peak at the wavelength of the absorption maximum of the enzyme (450 nm) when it is in the reduced state and complexed with carbon monoxide.
This cysteine and several flanking residues are highly conserved in known P450s, and have the formal PROSITE signature consensus pattern [FW] - [SGNH] - x - [GD] - {F} - [RKHPT] - {P} - C - [LIVMFAP] - [GAD].
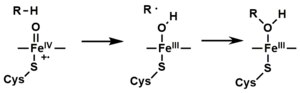
[8] In general, the P450 catalytic cycle proceeds as follows: Mechanistic details, including the oxygen rebound mechanism, have been investigated with synthetic analogues, consisting of iron oxo heme complexes.
The cysteine binds Fe and arginine, forming strong electrostatic interactions with negatively charged side chains of the heme.
The glycine residues within the conserved sequence are essential, as their small structure enables surrounding alpha helices to remain in place without interacting with a variant amino acid.