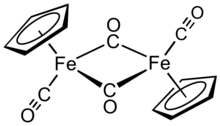
Cyclopentadienyliron dicarbonyl dimer
Cp2Fe2(CO)4 is reasonably stable to storage under air and serves as a convenient starting material for accessing other Fp (CpFe(CO)2) derivatives (described below).
[2] In solution, the cis, trans, and open isomers interconvert rapidly at room temperature, making the molecular structure fluxional.
This view is consistent with computations and X-ray crystallographic data that indicate a lack of significant electron density between the iron atoms.
This formalism is argued to give misleading implications with respect to the chemical and spectroscopic behavior of the carbonyl groups.
[7] Cp2Fe2(CO)4 was first prepared in 1955 at Harvard by Geoffrey Wilkinson using the same method employed today: the reaction of iron pentacarbonyl and dicyclopentadiene.
[3] Although of no major commercial value, Fp2 is a workhorse in organometallic chemistry because it is inexpensive and FpX derivatives are rugged (X = halide, organyl).
[CpFe(CO)2]Na is a widely studied reagent since it is readily alkylated, acylated, or metalated by treatment with an appropriate electrophile.
[11] Treatment of NaFp with an alkyl halide (RX, X = Br, I) produces FeR(η5-C5H5)(CO)2 Fp2 can also be cleaved with alkali metals[12] and by electrochemical reduction.
[13][14] Halogens oxidatively cleave [CpFe(CO)2]2 to give the Fe(II) species FpX (X = Cl, Br, I): One example is cyclopentadienyliron dicarbonyl iodide.
[15] In another approach, salts of [Fp(isobutene)]+ are readily obtained by reaction of NaFp with methallyl chloride followed by protonolysis.
Fp–allyl and Fp–allenyl react with cationic electrophiles E (such as Me3O+, carbocations, oxocarbenium ions) to generate allylic and propargylic functionalization products, respectively (eqn 2, shown for allyliron).
[22] η2-Allenyl complexes of Fp+ and substituted cyclopentadienyliron dicarbonyl cations have also been characterized, with X-ray crystallographic analysis showing substantial bending at the central allenic carbon (bond angle < 150°).
[25] The key reagent is prepared from FpNa with a thioether and methyl iodide, and has a good shelf-life, in contrast to typical Simmons-Smith intermediates and diazoalkanes.