Cheletropic reaction
The key distinguishing feature of cheletropic reactions is that on one of the reagents, both new bonds are being made to the same atom.
The reaction process can be shown using two different geometries, the small molecule can approach in a linear or non-linear fashion.
Shown below is a diagram of a two-electron fragment approaching a four-electron π-system using frontier molecular orbitals.
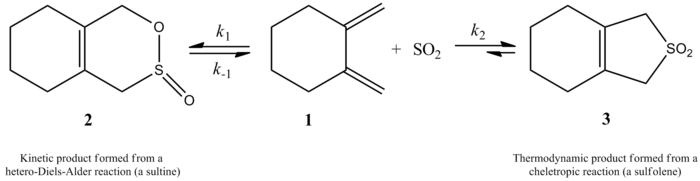
[1] In 1995, Suarez and Sordo showed that sulfur dioxide when reacted with butadiene and isoprene gives two different products depending on the mechanism.
The cheletropic pathway is favored because it gives rise to a more stable five-membered ring adduct.
Rates of addition were monitored in benzene at 30 °C with an initial twentyfold excess of sulfur dioxide, allowing for a pseudo first-order approximation.
The authors attribute this to the tendency of bulky groups to favor the cisoid conformation of the diene which is essential to the reaction (see table below).
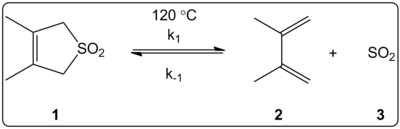
[5] More recently, a 2002 study by Monnat, Vogel, and Sordo measured the kinetics of addition of sulfur dioxide to 1,2-dimethylidenecycloalkanes.
[6] The authors were able to experimentally determine a rate law at 261.2 K for the reaction of 1,2-dimethylidenecyclohexane with sulfur dioxide to give the corresponding sulfolene.
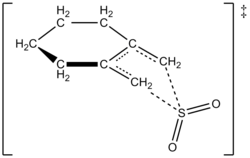
Using computational methods, the authors proposed a transition structure for the cheletropic reaction of 1,2-dimethylidenecyclohexane with sulfur dioxide (see figure at right).
It is suggested that there is a change of the polarity during the activation process as evidenced by correlations between the equilibrium and kinetic data.
The authors remark that the reaction appears to be influenced by the polarity of the solvent, and this can be explained by the change in the dipole moments when going from reactant to transition state to product.
The authors also state that the cheletropic reaction doesn’t seem to be influenced by either solvent acidity or basicity.
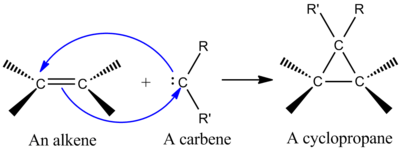
[8] One of the most synthetically important cheletropic reactions is the addition of a singlet carbene to an alkene to make a cyclopropane (see figure at left).

Case 1: the single atom is the carbonyl carbon ( C=O ) that ends up in carbon monoxide ( C≡O ).
Case 2: the single atom is the nitrogen atom in the diazenyl group ( N=N ), which ends up as dinitrogen ( N≡N ).
The above are known as cheletropic eliminations because a small, stable molecule is given off in the reaction. [ 1 ]
Case 3 & 4: the single atom is the sulfur in sulfur dioxide ( SO 2 ), which joins the alkene chains to form a ring.