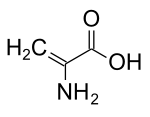
Dehydroalanine
It does not exist in its free form, but it occurs naturally as a residue found in peptides of microbial origin.
The required precursors are serine or cysteine residues, which undergo enzyme-mediated loss of water and hydrogen sulfide, respectively.
These are electrophilic due to the α,β-unsaturated carbonyl,[2] and can, for example, alkylate other amino acids.
DHA can be formed from cysteine or serine by simple base catalysis without the need for an enzyme, which can happen during cooking and alkaline food preparation processes.
It can then alkylate other amino acid residues, such as lysine, forming lysinoalanine cross-links and racemization of the original alanine.