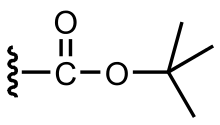
tert-Butyloxycarbonyl protecting group
The BOC group can be added to amines under aqueous conditions using di-tert-butyl dicarbonate in the presence of a base such as sodium hydroxide:
Protection of amines can also be accomplished in acetonitrile solution using 4-dimethylaminopyridine (DMAP) as the base.
[2][3][4] A complication may be the tendency of the t-butyl cation intermediate to alkylate other nucleophiles; scavengers such as anisole or thioanisole may be used.
[9] The mechanism involves silylation of the carbonyl oxygen and elimination of tert-butyl iodide (1), methanolysis of the silyl ester to the carbamic acid (2) and finally decarboxylation to the amine (3).
Upon deprotonation, this reagent affords a doubly BOC-protected source of NH−2, which can be N-alkylated.