Density
Mathematically, density is defined as mass divided by volume:[1]
In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume,[2] although this is scientifically inaccurate – this quantity is more specifically called specific weight.
Osmium is the densest known element at standard conditions for temperature and pressure.
This variation is typically small for solids and liquids but much greater for gases.
The reciprocal of the density of a substance is occasionally called its specific volume, a term sometimes used in thermodynamics.
Aristotle, for example, wrote:[3] There is so great a difference in density between salt and fresh water that vessels laden with cargoes of the same weight almost sink in rivers, but ride quite easily at sea and are quite seaworthy.
And an ignorance of this has sometimes cost people dear who load their ships in rivers.
For they say if you bind a man or beast and throw him into it he floats and does not sink beneath the surface.In a well-known but probably apocryphal tale, Archimedes was given the task of determining whether King Hiero's goldsmith was embezzling gold during the manufacture of a golden wreath dedicated to the gods and replacing it with another, cheaper alloy.
[4] Archimedes knew that the irregularly shaped wreath could be crushed into a cube whose volume could be calculated easily and compared with the mass; but the king did not approve of this.
Upon this discovery, he leapt from his bath and ran naked through the streets shouting, "Eureka!
As a result, the term eureka entered common parlance and is used today to indicate a moment of enlightenment.
The story first appeared in written form in Vitruvius' books of architecture, two centuries after it supposedly took place.
[5] Some scholars have doubted the accuracy of this tale, saying among other things that the method would have required precise measurements that would have been difficult to make at the time.
[6][7] Nevertheless, in 1586, Galileo Galilei, in one of his first experiments, made a possible reconstruction of how the experiment could have been performed with ancient Greek resources[8] From the equation for density (ρ = m/V), mass density has any unit that is mass divided by volume.
The density of precious metals could conceivably be based on Troy ounces and pounds, a possible cause of confusion.
Knowing the volume of the unit cell of a crystalline material and its formula weight (in daltons), the density can be calculated.
A number of techniques as well as standards exist for the measurement of density of materials.
To determine the density of a liquid or a gas, a hydrometer, a dasymeter or a Coriolis flow meter may be used, respectively.
If the body is not homogeneous, then its density varies between different regions of the object.
In the limit of an infinitesimal volume the density of an inhomogeneous object at a point becomes:
In practice, bulk materials such as sugar, sand, or snow contain voids.
Commonly the void is air, but it could also be vacuum, liquid, solid, or a different gas or gaseous mixture.
To determine the material volumetric mass density, one must first discount the volume of the void fraction.
If the material is under pressure (commonly ambient air pressure at the earth's surface) the determination of mass from a measured sample weight might need to account for buoyancy effects due to the density of the void constituent, depending on how the measurement was conducted.
For example, the density of water increases between its melting point at 0 °C and 4 °C; similar behavior is observed in silicon at low temperatures.
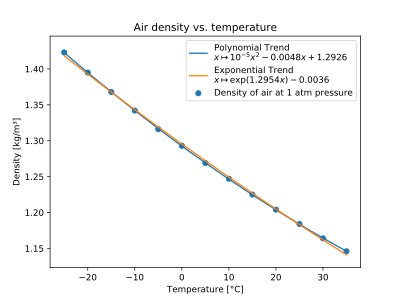
The effect of pressure and temperature on the densities of liquids and solids is small.
This roughly translates into needing around ten thousand times atmospheric pressure to reduce the volume of a substance by one percent.
(Although the pressures needed may be around a thousand times smaller for sandy soil and some clays.)
A one percent expansion of volume typically requires a temperature increase on the order of thousands of degrees Celsius.
where M is the molar mass, P is the pressure, R is the universal gas constant, and T is the absolute temperature.