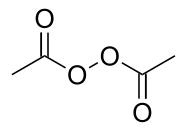
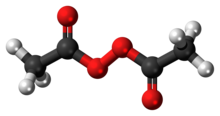
Diacetyl peroxide
It is a white solid or oily liquid with a sharp odor.
[2] Diacetyl peroxide was discovered in 1858 by Benjamin Collins Brodie,[6] who obtained the compound by treating glacial acetic acid with barium peroxide in anhydrous diethyl ether.
[8][9][2] The threshold quantity for Process Safety Management per Occupational Safety and Health Administration 1910.119 is 5,000 lb (2,300 kg) if the concentration of the diacetyl peroxide solution is greater than 70%.
[11] The crystalline peroxide is especially shock sensitive and a high explosion risk.
[12][9] Organic peroxides are all prone to exothermic decomposition, potentially leading to explosions and fire.