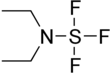Diethylaminosulfur trifluoride
DAST converts alcohols to the corresponding alkyl fluorides as well as aldehydes and unhindered ketones to geminal difluorides.
Sulfur tetrafluoride, SF4, effects the same transformation but will also convert the acyl fluoride to the trifluoromethyl derivative.
[2] Acid-labile substrates are less likely to undergo rearrangement and elimination since DAST is less prone to contamination with acids.
DAST is prepared by the reaction of diethylaminotrimethylsilane and sulfur tetrafluoride:[3] The original paper calls for trichlorofluoromethane (Freon-11) as a solvent.
[5] Because of the dangers involved in the preparation of DAST (glass etching, possibility of exothermic events), it is often purchased from a commercial source.