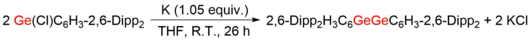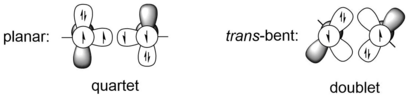Digermyne
Because of the large interatomic repulsion between two Ge atoms, only kinetically stabilized digermyne molecules can be synthesized and characterized by utilizing bulky protecting groups and appropriate synthetic methods, for example, reductive coupling of germanium(II) halides.
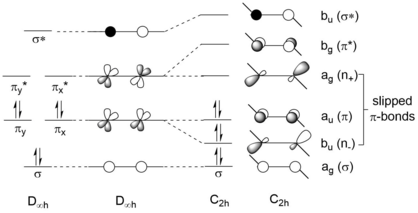
[1] The second order Jahn-Teller (SOJT) effect of digermynes gives rise to slipped π-bond and large molecular geometrical distortion.
Although many computational studies have calculated the structures and energies of the parent molecule HGeGeH[2][3] and digermynes with organic substitutes,[4] they can be only synthesized and isolated upon the protection of bulky R groups.
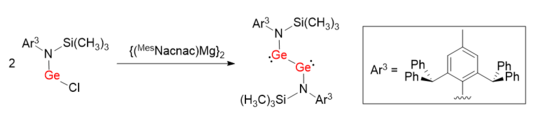
It has been proven that the synthetic strategy that reducing proper precursor, usually germanium(II) halides with bulky protection groups, by strong reductants is powerful for synthesizing digermynes.
Similar molecule, designated Ar2GeGeAr2 has been calculated before the characterization of Ar1GeGeAr1, with the optimized trans-bent core structure protected by even more crowded 2,6-Trip2C6H2 (Ar2, Trip = 2,4,6-triisopropylphenyl) groups.
The abnormal bond angles and the single-bond feature of LGeGeL can be rationalized by the electron donating character of N atom, which leads to the formation of N p(π)→Ge (empty p orbital) interaction.
[8] In a molecular orbital (MO) description, the geometrical distortion (trans-bent structure) of digermynes is the consequence of the second order Jahn-Teller (SOJT) effect, which is the symmetry allowed interaction between filled bonding MO (generally the HOMO in digermynes) and empty nonbonding or antibonding MOs (usually the latter one) that are close in energy and can lead to large molecular distortion.
Digermynes are able to react with a variety of unsaturated small molecules, including alkynes, alkenes, PhN=NPh, isocyanides, and azides, due to their relatively weak Ge-Ge bonds.
Ge atom of the germirane substituent then easily inserts into one of the Ge-C bonds of germylene to generate 1,2-digermacyclobutene, which has been illustrated both experimentally[13] and computationally.
The increased steric repulsion of two GeC4 rings leads to the homolytic cleavage of the Ge-Ge single bond which then produces the final germane by 1,4-addition reaction with additional equivalent of the 2,3-dimethyl-1,3-butadiene.
[8] BbtGeGeBbt has been proven to be able to undergo addition reaction with alcohols such as methanol and water to generate 1,1-dimethoxydigermane and 1,1-dihydroxydigermane, respectively, which demonstrate the multiple-bond character of digermynes.