Diglyme
It is prepared by a reaction of dimethyl ether and ethylene oxide over an acid catalyst.
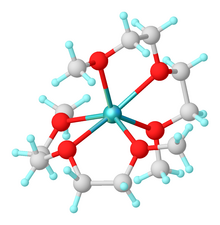
[2] Because of its resistance to strong bases, diglyme is favored as a solvent for reactions of alkali metal reagents even at high temperatures.
[6][7] It serves as a chelate for alkali metal cations, leaving anions more active.
The European Chemicals Agency lists diglyme as a substance of very high concern (SVHC) as a reproductive toxin.
[8] At higher temperatures and in the presence of active metals diglyme is known to decompose, which can produce large amounts of gas and heat.