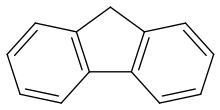
Fluorene
It forms white crystals that exhibit a characteristic, aromatic odor similar to that of naphthalene.
For commercial purposes it is obtained from coal tar,[3] where it was discovered and named by Marcellin Berthelot in 1867.
[8] Fluorene can be found after the incomplete combustion of plastics such as PS, PE and PVC.
[10]) Deprotonation gives the stable fluorenyl anion, nominally C13H9−, which is aromatic and has an intense orange colour.
The purification of fluorene exploits its acidity and the low solubility of its sodium derivative in hydrocarbon solvents.