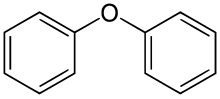
Diphenyl ether
[6] Now it is synthesized by a modification of the Williamson ether synthesis, here the reaction of phenol and bromobenzene in the presence of base and a catalytic amount of copper: Involving similar reactions, diphenyl ether is a significant side product in the high-pressure hydrolysis of chlorobenzene in the production of phenol.
[8] The compound undergoes reactions typical of other phenyl rings, including hydroxylation, nitration, halogenation, sulfonation, and Friedel–Crafts alkylation or acylation.
Such a mixture is well-suited for heat transfer applications because of the relatively large temperature range of its liquid state.
[12] Because of its odor reminiscent of scented geranium, as well as its stability and low price, diphenyl ether is used widely in soap perfumes.
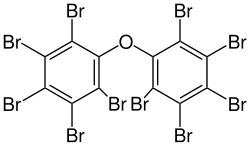
Decabromodiphenyl oxide is sold under the trade name Saytex 102 as a flame retardant in the manufacture of paints and reinforced plastics.