Doping (semiconductor)
The effects of impurities in semiconductors (doping) were long known empirically in such devices as crystal radio detectors and selenium rectifiers.
For instance, in 1885 Shelford Bidwell, and in 1930 the German scientist Bernhard Gudden, each independently reported that the properties of semiconductors were due to the impurities they contained.
[1][2] A doping process was formally developed by John Robert Woodyard working at Sperry Gyroscope Company during World War II.
Though the word doping is not used in it, his US Patent issued in 1950 describes methods for adding tiny amounts of solid elements from the nitrogen column of the periodic table to germanium to produce rectifying devices.
Typical concentration values fall somewhere in this range and are tailored to produce the desired properties in the device that the semiconductor is intended for.
Because EB is so small, room temperature is hot enough to thermally ionize practically all of the dopant atoms and create free charge carriers in the conduction or valence bands.
The concentration factors NC(T) and NV(T) are given by where me* and mh* are the density of states effective masses of electrons and holes, respectively, quantities that are roughly constant over temperature.
[7] Some dopants are added as the (usually silicon) boule is grown by Czochralski method, giving each wafer an almost uniform initial doping.
For example, in the case of n-type gas doping of gallium arsenide, hydrogen sulfide is added, and sulfur is incorporated into the structure.
[10] In the case of semiconductors in general, only a very thin layer of the wafer needs to be doped in order to obtain the desired electronic properties.
NTD is a far less common doping method than diffusion or ion implantation, but it has the advantage of creating an extremely uniform dopant distribution.
Boron is the p-type dopant of choice for silicon integrated circuit production because it diffuses at a rate that makes junction depths easily controllable.
By doping pure silicon with Group V elements such as phosphorus, extra valence electrons are added that become unbounded from individual atoms and allow the compound to be an electrically conductive n-type semiconductor.
Doping with Group III elements, which are missing the fourth valence electron, creates "broken bonds" (holes) in the silicon lattice that are free to move.
A very heavily doped semiconductor behaves more like a good conductor (metal) and thus exhibits more linear positive thermal coefficient.
If an equal number of donors and acceptors are present in the semiconductor, the extra core electrons provided by the former will be used to satisfy the broken bonds due to the latter, so that doping produces no free carriers of either type.
Partial compensation, where donors outnumber acceptors or vice versa, allows device makers to repeatedly reverse (invert) the type of a certain layer under the surface of a bulk semiconductor by diffusing or implanting successively higher doses of dopants, so-called counterdoping.
Most modern semiconductor devices are made by successive selective counterdoping steps to create the necessary P and N type areas under the surface of bulk silicon.
[29] However, similar to the problem encountered in doping conductive polymers, air-stable n-dopants suitable for materials with low electron affinity (EA) are still elusive.
Recently, photoactivation with a combination of cleavable dimeric dopants, such as [RuCp∗Mes]2, suggests a new path to realize effective n-doping in low-EA materials.
The presence of disperse ferromagnetic species is key to the functionality of emerging spintronics, a class of systems that utilise electron spin in addition to charge.
Using density functional theory (DFT) the temperature dependent magnetic behaviour of dopants within a given lattice can be modeled to identify candidate semiconductor systems.
[32] The sensitive dependence of a semiconductor's properties on dopants has provided an extensive range of tunable phenomena to explore and apply to devices.
New applications have become available that require the discrete character of a single dopant, such as single-spin devices in the area of quantum information or single-dopant transistors.
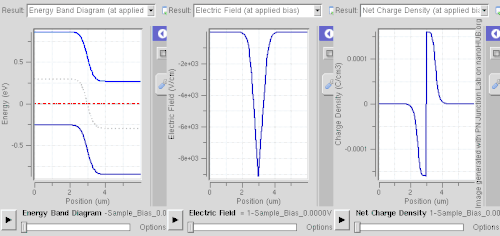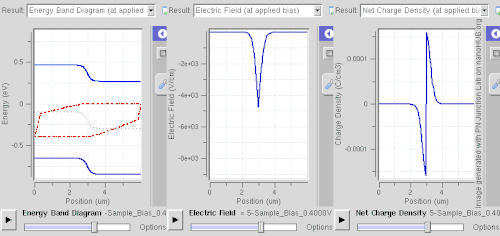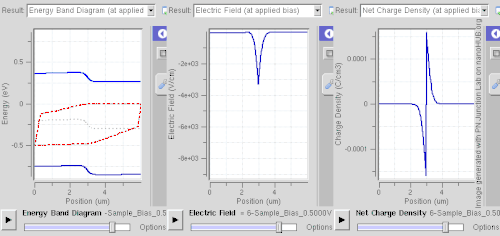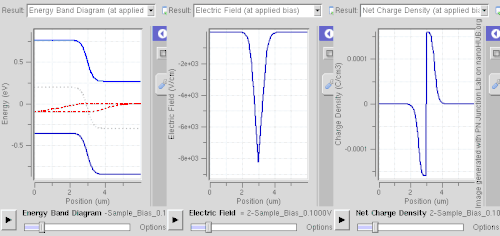
Ionized donors and acceptors however attract electrons and holes, respectively, so this spatial separation requires abrupt changes of dopant levels, of band gap (e.g. a quantum well), or built-in electric fields (e.g. in case of noncentrosymmetric crystals).
This technique is called modulation doping and is advantageous owing to suppressed carrier-donor scattering, allowing very high mobility to be attained.