Double-layer capacitance
Double-layer capacitance is the important characteristic of the electrical double layer[1][2] which appears at the interface between a surface and a fluid (for example, between a conductive electrode and an adjacent liquid electrolyte).
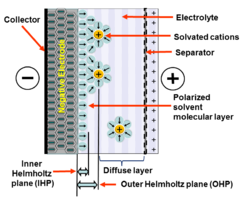
Where the liquid electrolyte contacts the electrode's conductive metallic surface, an interface is formed which represents a common boundary between the two phases of matter.
It adheres by physical adsorption on the electrode surface and separates the oppositely polarized ions from each other, forming a molecular dielectric.
The amount of charge in the electrode is matched by the magnitude of counter-charges in the outer Helmholtz plane (OHP).
This separation of two layers of polarized ions through the double-layer stores electrical charges in the same way as in a conventional capacitor.
The double-layer charge forms a static electric field in the molecular IHP layer of the solvent molecules that corresponds to the strength of the applied voltage.
The magnitude of the electric charge that can accumulate in the layers corresponds to the concentration of the adsorbed ions and the electrodes surface.
Because activated carbon electrodes have a very high surface area and an extremely thin double-layer distance which is on the order of a few ångströms (0.3-0.8 nm), it is understandable why supercapacitors have the highest capacitance values among the capacitors (in the range of 10 to 40 μF/cm2).
Parameters such as electrode material and structure, electrolyte mixture, and amount of pseudocapacitance also contribute to capacitance value.