Pseudocapacitance
Pseudocapacitance is the electrochemical storage of electricity in an electrochemical capacitor that occurs due to faradaic charge transfer originating from a very fast sequence of reversible faradaic redox, electrosorption or intercalation processes on the surface of suitable electrodes.
[1][2][3] Pseudocapacitance is accompanied by an electron charge-transfer between electrolyte and electrode coming from a de-solvated and adsorbed ion.
Redox reactions in batteries with faradaic charge-transfer between an electrolyte and the surface of an electrode were characterized decades ago.
Compared with batteries, supercapacitor faradaic processes are much faster and more stable over time, because they leave only traces of reaction products.
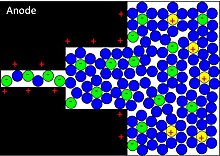
Accompanied by the electric double-layer, some de-solvated electrolyte ions pervade the separating solvent layer and are adsorbed by the electrode's surface atoms.
This faradaic charge transfer, originated by a fast sequence of reversible redox reactions, electrosorptions or intercalation processes between electrolyte and the electrode surface is called pseudocapacitance.
[8] Depending on the electrode's structure or surface material, pseudocapacitance can originate when specifically adsorbed ions pervade the double-layer, proceeding in several one-electron stages.
This kind of pseudocapacitance has a linear function within narrow limits and is determined by the potential-dependent degree of surface coverage of the adsorbed anions.
[8][9] When discharging pseudocapacitance, the charge transfer is reversed and the ions or atoms leave the double-layer and spread throughout the electrolyte.
[citation needed] Ruthenium dioxide (RuO2) in combination with sulfuric acid (H2SO4) electrolyte provides one of the best examples of pseudocapacitance, with a charge/discharge over a window of about 1.2 V per electrode.
Pseudocapacitance originates from a coupled, reversible redox reaction with several oxidation steps with overlapping potential.
The electron transfer reaction takes place according to: During charge and discharge, H+ (protons) are incorporated into or removed from the RuO2 crystal lattice, which generates storage of electrical energy without chemical transformation.
Conductive polymer such as polyaniline, polythiophene, polypyrrole and polyacetylene have a lower reversibility of the redox processes involving faradaic charge transfer than transition metal oxides, and suffer from a limited stability during cycling.
[citation needed] Such electrodes employ electrochemical doping or dedoping of the polymers with anions and cations.
The tailored sizes of pores in nano-structured carbon electrodes can maximize ion confinement, increasing specific capacitance by faradaic H2 adsorption treatment.
[15][16] Brezesinki et al. showed that mesoporous films of α-MoO3 have improved charge storage due to lithium ions inserting into the gaps of α-MoO3.