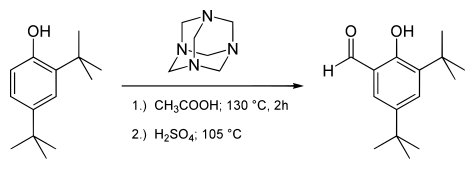
Duff reaction
[2] The reaction requires strongly electron donating substituents on the aromatic ring such as in a phenol.
If both ortho positions are vacant then a diformylation is possible, as in the formation of diformylcresol from p-cresol.
Addition to the aromatic ring results in an intermediate at the oxidation state of a benzylamine.
An intramolecular redox reaction then ensues, raising the benzylic carbon to the oxidation state of an aldehyde.
The oxygen atom is provided by water on acid hydrolysis in the final step.