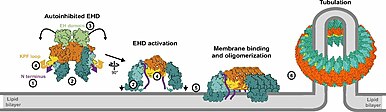
EHD protein family
This family is recognized by its highly conserved EH (Eps15 homology)[1] domain, a structural motif that has been shown to facilitate specificity and interaction between protein and ligand.
During the late 20th century, several advances were made regarding the identification of proteins involved in endocytotic recycling and other mechanisms of intracellular trafficking.
Rab proteins have been found to play a major role in endocytotic recycling via SNARE-based vesicle fusion and transport.
When bound to GTP, Rab proteins have a large affinity for their respective effectors which then work to carry out a specific function.
Current research suggests that the EH domain interacts with the NPF motif, a basic region classified by its arginine (N), proline (P), and phenylalanine (F) constituents.
[4] The two helical domains act as lipid binding interfaces so that the EHD protein can interact with the cell membrane.
Fast recycling is a direct pathway from early endosome to the cell membrane without an intermediate organelle present.
Evidence for this is implied as present-day research has only observed the consequences of the depletion of EHD3 concentration levels which renders transport from early endosome to ERC defective.