Embryonic stem cell
[3][4] Researchers are currently focusing heavily on the therapeutic potential of embryonic stem cells, with clinical use being the goal for many laboratories.
ESCs have a normal karyotype, maintain high telomerase activity, and exhibit remarkable long-term proliferative potential.
[10] Retinoblastoma proteins that inhibit the transcription factor E2F until the cell is ready to enter S phase are hyperphosphorylated and inactivated in ESCs, leading to continual expression of proliferation genes.
[15][16] Due to their plasticity and potentially unlimited capacity for self-renewal, embryonic stem cell therapies have been proposed for regenerative medicine and tissue replacement after injury or disease.
Pluripotent stem cells have shown promise in treating a number of varying conditions, including but not limited to: spinal cord injuries, age related macular degeneration, diabetes, neurodegenerative disorders (such as Parkinson's disease), AIDS, etc.
It is theorized that if embryonic stem cells can be altered to not evoke the immune response when implanted into the patient then this would be a revolutionary step in tissue engineering.
[22] Besides becoming an important alternative to organ transplants, ESCs are also being used in the field of toxicology, and as cellular screens to uncover new chemical entities that can be developed as small-molecule drugs.
Studies have shown that cardiomyocytes derived from ESCs are validated in vitro models to test drug responses and predict toxicity profiles.
[21] ESC derived cardiomyocytes have been shown to respond to pharmacological stimuli and hence can be used to assess cardiotoxicity such as torsades de pointes.
This approach may very well prove valuable at studying disorders such as Fragile-X syndrome, Cystic fibrosis, and other genetic maladies that have no reliable model system.
[35] Apoptosis can be used as a fail-safe strategy to remove cells with un-repaired DNA damages in order to avoid mutation and progression to cancer.
[38] The study leading to this scientific advancement was conducted by Hans Keirstead and colleagues at the University of California, Irvine and supported by Geron Corporation of Menlo Park, CA, founded by Michael D. West, PhD.
A previous experiment had shown an improvement in locomotor recovery in spinal cord-injured rats after a 7-day delayed transplantation of human ESCs that had been pushed into an oligodendrocytic lineage.
[39] The phase I clinical study was designed to enroll about eight to ten paraplegics who have had their injuries no longer than two weeks before the trial begins, since the cells must be injected before scar tissue is able to form.
This first trial was primarily designed to test the safety of these procedures and if everything went well, it was hoped that it would lead to future studies that involve people with more severe disabilities.
[44] BioTime company Asterias Biotherapeutics (NYSE MKT: AST) was granted a $14.3 million Strategic Partnership Award by the California Institute for Regenerative Medicine (CIRM) to re-initiate the world's first embryonic stem cell-based human clinical trial, for spinal cord injury.
Supported by California public funds, CIRM is the largest funder of stem cell-related research and development in the world.
OPCs and their mature derivatives called oligodendrocytes provide critical functional support for nerve cells in the spinal cord and brain.
Asterias recently presented the results from phase 1 clinical trial testing of a low dose of AST-OPC1 in patients with neurologically complete thoracic spinal cord injury.
Patients followed 2–3 years after AST-OPC1 administration showed no evidence of serious adverse events associated with the cells in detailed follow-up assessments including frequent neurological exams and MRIs.
[45] The major concern with the possible transplantation of ESCs into patients as therapies is their ability to form tumors including teratomas.
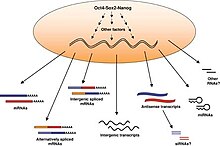
[48] Later protocols to induce pluripotency bypass these problems completely by using non-integrating RNA viral vectors such as sendai virus or mRNA transfection.
Martin Evans and Matthew Kaufman reported a technique that delays embryo implantation, allowing the inner cell mass to increase.
This process includes removing the donor mother's ovaries and dosing her with progesterone, changing the hormone environment, which causes the embryos to remain free in the uterus.
However, as a first indication that the iPS cell technology can in rapid succession lead to new cures, it was used by a research team headed by Rudolf Jaenisch of the Whitehead Institute for Biomedical Research in Cambridge, Massachusetts, to cure mice of sickle cell anemia, as reported by Science journal's online edition on December 6, 2007.
[77][78] On January 16, 2008, a California-based company, Stemagen, announced that they had created the first mature cloned human embryos from single skin cells taken from adults.
The problem was discovered when non-human sialic acid in the growth medium was found to compromise the potential uses of the embryonic stem cells in humans, according to scientists at the University of California, San Diego.
[81] However, a study published in the online edition of Lancet Medical Journal on March 8, 2005, detailed information about a new stem cell line that was derived from human embryos under completely cell- and serum-free conditions.
After more than 6 months of undifferentiated proliferation, these cells demonstrated the potential to form derivatives of all three embryonic germ layers both in vitro and in teratomas.
[83] Muse cells reside in the connective tissue of nearly every organ including the umbilical cord, bone marrow and peripheral blood.