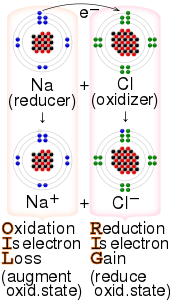
Electron transfer
ET reactions are relevant to photosynthesis and respiration and commonly involve transition metal complexes.
More commonly, however, the covalent linkage is transitory, forming just prior to the ET and then disconnecting following the ET event.
A famous example of an inner sphere ET process that proceeds via a transitory bridged intermediate is the reduction of [CoCl(NH3)5]2+ by [Cr(H2O)6]2+.
[7] In outer-sphere ET reactions, the participating redox centers are not linked via any bridge during the ET event.
As an example, self-exchange describes the degenerate reaction between permanganate and its one-electron reduced relative manganate: In general, if electron transfer is faster than ligand substitution, the reaction will follow the outer-sphere electron transfer route.
Theories addressing heterogeneous electron transfer have applications in electrochemistry and the design of solar cells.
Both theories are, however, semiclassical in nature, although they have been extended to fully quantum mechanical treatments by Joshua Jortner, Alexander M. Kuznetsov, and others proceeding from Fermi's golden rule and following earlier work in non-radiative transitions.