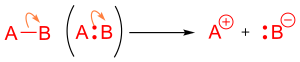Arrow pushing
The arrows illustrate the movement of electrons as bonds between atoms are broken and formed.
Arrow pushing is also used to describe how positive and negative charges are distributed around organic molecules through resonance.
[2] The representation of reaction mechanisms using curved arrows to indicate electron flow was developed by Sir Robert Robinson in 1922.
[3][4][5] Organic chemists use two types of arrows within molecular structures to describe electron movements.
Organic chemists represent the formation of a bond by a curved arrow pointing between two species.
Some authorities[1] allow the simplification that an arrow can originate at a formal negative charge that corresponds to a lone pair.
However, not all formal negative charges correspond to the presence of a lone pair (e.g., the B in F4B−), and care needs to be taken with this usage.
A covalent bond joining atoms in an organic molecule consists of a group of two electrons.
The consequence of this process is the retention of a single unpaired electron denoted by a dot on each of the atoms that were formerly joined by a bond.
The single electron movement can be denoted by a curved arrow commonly referred to as a fish hook.
After the reaction occurs, it leads to both chlorine molecules left with a single unpaired electron.
This is because the most electronegative atom will naturally attract electrons towards itself more strongly, leading to its negative charge.
In a reaction involving Brønsted-Lowry acids and bases, the arrows are used in the same manner, and they help to indicate the attacking proton.
Because this mechanism proceeds with the interaction of two species at the transition state, it is referred to as a bimolecular process, resulting in the SN2 designation.
An E1 elimination occurs when a proton adjacent to a positive charge leaves and generates a double bond.
Because this mechanism proceeds with the initial dissociation of a single starting material forming a carbocation, this process is considered a uni-molecular reaction.
In general, these reactions take place when esters (or related functional groups) react with nucleophiles.