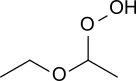
Diethyl ether peroxide
Diethyl ether hydroperoxide and its condensation products are responsible for the explosive organic peroxides that slowly form upon exposure of diethyl ether to ambient air and temperature conditions.
This is a radical process, driven by UV excitation of molecular oxygen into a more reactive form.
It can be intentionally prepared in high yield by the acid-catalyzed addition of hydrogen peroxide to ethyl vinyl ether:[1] Related hydroperoxides can be produced similarly.
The peroxide is a colorless oil that is an extremely brisant and friction sensitive explosive material, however the polymeric materials are solid making them more dangerous as evaporation of the volatile diethyl ether can leave thin films of pure explosive.
A positive test results in the formation of iodine (I2) that causes a yellow or brown color of the ether phase or a dark bluish spot on the paper strip.