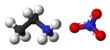
Ethylammonium nitrate
[4] This compound was described by Paul Walden in 1914,[5][6] and is believed to be the earliest reported example of a room-temperature ionic liquid.
[9] The ethylammonium ion (CH3CH2NH+3) has three easily detachable protons which are tetrahedrally arranged around the central nitrogen atom, whereas the configuration of the NO−3 anion is planar.
Despite the structural differences, EAN shares many properties with water, such as micelle formation, aggregation of hydrocarbons, negative enthalpy and entropy of dissolution of gases, etc.
[10] Ethylammonium nitrate is used as an electrically conductive solvent in electrochemistry and as a protein crystallization agent.
The refolding action was explained as follows: The ethyl group of ethylammonium nitrate interacts with the hydrophobic part of the protein and thereby protects it from intermolecular association, whereas the charged part of EAN stabilizes the electrostatic interactions.