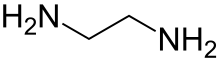
Ethylenediamine
Ethylenediamine is produced industrially by treating 1,2-dichloroethane with ammonia under pressure at 180 °C in an aqueous medium:[6][7] In this reaction hydrogen chloride is generated, which forms a salt with the amine.
It can be prepared in the lab by the reaction of either ethylene glycol or ethanolamine and urea, followed by decarboxylation of the ethyleneurea intermediate.
[11] Salts of ethylenebisdithiocarbamate are commercially significant fungicides under the brand names Maneb, Mancozeb, Zineb, and Metiram.
[12] When used as a pharmaceutical excipient, after oral administration its bioavailability is about 0.34, due to a substantial first-pass effect.
The derivative N,N-ethylenebis(stearamide) (EBS) is a commercially significant mold-release agent and a surfactant in gasoline and motor oil.
Related derivatives of ethylenediamine include ethylenediaminetetraacetic acid (EDTA), tetramethylethylenediamine (TMEDA), and tetraethylethylenediamine (TEEDA).
Unless tightly contained, liquid ethylenediamine will release toxic and irritating vapors into its surroundings, especially on heating.
The vapors absorb moisture from humid air to form a characteristic white mist, which is extremely irritating to skin, eyes, lungs and mucous membranes.