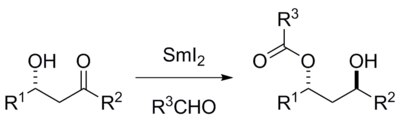
Evans–Tishchenko reaction
It was first described in 1990 by David A. Evans and Amir Hoveyda, as a development of the well-known Tishchenko reaction discovered in 1906.
[2] The reaction mechanism involves the attack of the aldehyde from the free alcohol group.
The Lewis acid can then chelate between the two oxygen atoms to form a cyclic, 6-membered transition state.
The hydride, formerly the formyl hydrogen on the aldehyde, is delivered intramolecularly, accounting for the observed anti diastereoselectivity: the result is a 1,3-anti diol monoester.
The proposed mechanism is further supported by isotopic labeling, which demonstrates that the formyl hydrogen is the one that migrates.