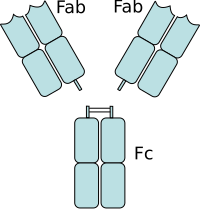
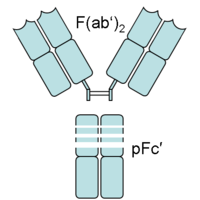
Fragment crystallizable region
This region allows antibodies to activate the immune system, for example, through binding to Fc receptors.
[1][2] The Fc regions of IgGs bear a highly conserved N-glycosylation site.
[5] The N-glycans attached to this site are predominantly core-fucosylated diantennary structures of the complex type.
In addition, small amounts of these N-glycans also bear bisecting GlcNAc and α-2,6 linked sialic acid residues.
[6] In a new development in the field of antibody-based therapeutics, the Fc region of immunoglobulins has been engineered to contain an antigen-binding site.