Mast cell
[7] The first in vitro differentiation and growth of a pure population of mouse mast cells was carried out using conditioned medium derived from concanavalin A-stimulated splenocytes.
[8] Later, it was discovered that T cell-derived interleukin 3 was the component present in the conditioned media that was required for mast cell differentiation and growth.
[7] Mast cells express a high-affinity receptor (FcεRI) for the Fc region of IgE, the least-abundant member of the antibodies.
Other membrane activation events can either prime mast cells for subsequent degranulation or act in synergy with FcεRI signal transduction.
The allergen binds to the antigen-binding sites, which are situated on the variable regions of the IgE molecules bound to the mast cell surface.
The clustering of the intracellular domains of the cell-bound Fc receptors, which are associated with the cross-linked IgE molecules, causes a complex sequence of reactions inside the mast cell that lead to its activation.
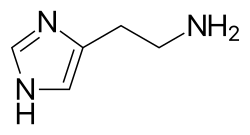
[12] Examples of mediators that are released into the extracellular environment during mast cell degranulation include:[7][12][16] Histamine dilates post-capillary venules, activates the endothelium, and increases blood vessel permeability.
The bump and redness immediately following a mosquito bite are a good example of this reaction, which occurs seconds after challenge of the mast cell by an allergen.
Several lines of evidence suggest that mast cells may have a fairly fundamental role in innate immunity: They are capable of elaborating a vast array of important cytokines and other inflammatory mediators such as TNF-α; they express multiple "pattern recognition receptors" thought to be involved in recognizing broad classes of pathogens; and mice without mast cells seem to be much more susceptible to a variety of infections.
[4] In the brain, mast cells are located in a number of structures that mediate visceral sensory (e.g. pain) or neuroendocrine functions or that are located along the blood–cerebrospinal fluid barrier, including the pituitary stalk, pineal gland, thalamus, and hypothalamus, area postrema, choroid plexus, and in the dural layer of the meninges near meningeal nociceptors.
[4] Mast cells serve the same general functions in the body and central nervous system, such as effecting or regulating allergic responses, innate and adaptive immunity, autoimmunity, and inflammation.
[19][20] In the gastrointestinal tract, mucosal mast cells are located in close proximity to sensory nerve fibres, which communicate bidirectionally.
[21] Neuronal activation induces neuropeptide (substance P and calcitonin gene-related peptide) signaling to mast cells where they bind to their associated receptors and trigger degranulation of a distinct set of mediators (β-Hexosaminidase, cytokines, chemokines, PGD2, leukotrienes, and eoxins).
This is due to the α chain containing endoplasmic reticulum retention signals that causes the α-chains to remain degraded in the ER.
The antigen cross-links the FcεR1 molecules, and Lyn tyrosine kinase phosphorylates the ITAMs in the FcεR1 β and γ chain in the cytoplasm.
[24] Phospholipase C gamma (PLCγ) becomes phosphorylated once bound to LAT, and is then used to catalyze phosphatidylinositol bisphosphate breakdown to yield inositol trisphosphate (IP3) and diacyglycerol (DAG).
Rab3 guanosine triphosphatases and Rab-associated kinases and phosphatases regulate granule membrane fusion in resting mast cells.
Human mast-cell-specific G-protein-coupled receptor MRGPRX2 plays a key role in the recognition of pathogen associated molecular patterns (PAMPs) and initiating an antibacterial response.
MRGPRX2 is able to bind to competence stimulating peptide (CSP) 1 - a quorum sensing molecule (QSM) produced by Gram-positive bacteria.
This might particularly be the case during Bartonella chronic infections where it appears clearly in human symptomatology that these patients all have a mast cell activation syndrome due to the presence of a not yet defined quorum sensing molecule (basal histamine itself?).
These patients also show cyclical skin pathergy and dermographism, every time the bacteria exits its hidden intracellular location.
[30] Recently, IgE-independent "pseudo-allergic" reactions are thought to also be mediated via the MRGPRX2 receptor activation of mast cells (e.g. drugs such as muscle relaxants, opioids, Icatibant and fluoroquinolones).
[36][37] Products released from these granules include histamine, serotonin, heparin, chondroitin sulphate, tryptase, chymase, carboxypeptidase, and TNF-α.
They have been shown to be involved in the recruitment of inflammatory cells to the joints (e.g., rheumatoid arthritis) and skin (e.g., bullous pemphigoid), and this activity is dependent on antibodies and complement components.
[40][41] This mutation, as well as expression of either CD2 or CD25 (confirmed by immunostaining or flow cytometry), are characteristic of primary clonal/monoclonal mast cell activation syndrome (CMCAS/MMAS).
[30][31] The syndrome is diagnosed based upon four sets of criteria involving treatment response, symptoms, a differential diagnosis, and biomarkers of mast cell degranulation.
[30][31] Mast cells were first described by Paul Ehrlich in his 1878 doctoral thesis on the basis of their unique staining characteristics and large granules.
These granules also led him to the incorrect belief that they existed to nourish the surrounding tissue, so he named them Mastzellen (from German Mast 'fattening', as of animals).