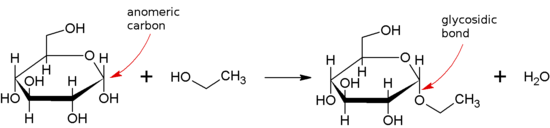
Glycosidic bond
C-glycosyl bonds have the glycosidic oxygen replaced by a carbon; the term "C-glycoside" is considered a misnomer by IUPAC and is discouraged.
[2] Pharmacologists often join substances to glucuronic acid via glycosidic bonds in order to increase their water solubility; this is known as glucuronidation.
[3][4][5] Employing a microwave oven equipped with refluxing apparatus in a rotor reactor with pressure bombs, Nüchter et al. (2001) were able to achieve 100% yield of α- and β-D-glucosides.
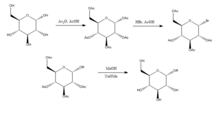
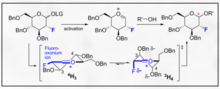
Joshi et al. (2006)[6] propose the Koenigs-Knorr reaction in the stereoselective synthesis of alkyl D-glucopyranosides via glycosylation, with the exception of using lithium carbonate which is less expensive and toxic than the conventional method of using silver or mercury salts.
On addition of the alcohol ROH and lithium carbonate, the OR replaces the bromine and on deprotecting the acetylated hydroxyls the product is synthesized in relatively high purity.
It was suggested by Joshi et al. (2001) that lithium acts as the nucleophile that attacks the carbon at the 5-position and through a transition state the alcohol is substituted for the bromine group.
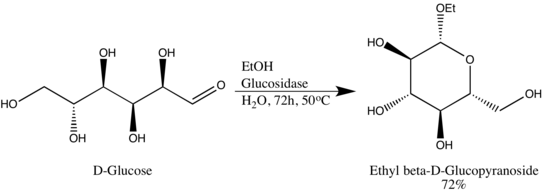
This specificity allows researchers to obtain glycosides in high epimeric excess, one example being Wen-Ya Lu's conversion of D-Glucose to Ethyl β-D-glucopyranoside using naturally-derived glucosidase.
The former often needs expensive materials and the later often shows low yields, De Winter et al.[10] investigated use of cellobiose phosphorylase (CP) toward synthesis of alpha-glycosides in ionic liquids.
O-linked glycopeptides recently have been shown to exhibit excellent CNS permeability and efficacy in multiple animal models with disease states.
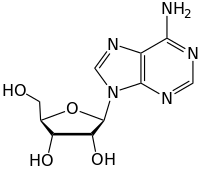
The nitrogen atoms from the amino group in the nucleotides are covalently linked to the anomeric carbon of the ribose sugar structure through an N-glycosidic bond.
Occasionally, the nucleobases attached to the ribose undergo deamination, alkylation, or oxidation which results in cytotoxic lesions along the DNA backbone.
The stepwise function, the nucleobase acts as a leaving group before the anomeric carbon gets attacked by the water molecule, producing a short-lived unstable oxacarbenium ion intermediate.