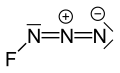Fluorine azide
[2] The bond between the fluorine atom and the nitrogen is very weak, leading to this substance being very unstable and prone to explosion.
[3] Calculations show the F–N–N angle to be around 102° with a straight line of 3 nitrogen atoms.
[5][7] Fluorine azide decomposes without explosion at normal temperatures to make dinitrogen difluoride: At higher temperatures such as 1000 °C fluorine azide breaks up into nitrogen monofluoride radical:[7] The FN itself dimerizes on cooling.
[8] Due to the explosion hazard, only very small quantities of this substance should be handled at a time.
[9] FN3 adducts can be formed with the Lewis acids boron trifluoride (BF3) and arsenic pentafluoride (AsF5) at -196 °C.