Fluorine
Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts.
Industrial production of fluorine gas for uranium enrichment, its largest application, began during the Manhattan Project in World War II.
Fluorine has no known metabolic role in mammals; a few plants and marine sponges synthesize organofluorine poisons (most often monofluoroacetates) that help deter predation.
[34][35] Oxygen does not combine with fluorine under ambient conditions, but can be made to react using electric discharge at low temperatures and pressures; the products tend to disintegrate into their constituent elements when heated.
[note 6] Andreas Sigismund Marggraf first characterized it in 1764 when he heated fluorite with sulfuric acid, and the resulting solution corroded its glass container.
[82] He also proposed in a letter to Sir Humphry Davy dated August 26, 1812 that this then-unknown substance may be named fluorine from fluoric acid and the -ine suffix of other halogens.
[77][87][88] Frémy's former student Henri Moissan persevered, and after much trial and error found that a mixture of potassium bifluoride and dry hydrogen fluoride was a conductor, enabling electrolysis.
[88][90] In 1906, two months before his death, Moissan received the Nobel Prize in Chemistry,[91] with the following citation:[87] [I]n recognition of the great services rendered by him in his investigation and isolation of the element fluorine ...
[80][92][93][94] Polytetrafluoroethylene (Teflon) was serendipitously discovered in 1938 by Roy J. Plunkett while working on refrigerants at Kinetic, and its superlative chemical and thermal resistance lent it to accelerated commercialization and mass production by 1941.
Since UF6 is as corrosive as fluorine, gaseous diffusion plants required special materials: nickel for membranes, fluoropolymers for seals, and liquid fluorocarbons as coolants and lubricants.
These compounds share many properties with perfluorocarbons such as stability and hydrophobicity,[162] while the functional group augments their reactivity, enabling them to adhere to surfaces or act as surfactants.
[164] Polytetrafluoroethylene (PTFE), the simplest fluoropolymer and perfluoro analogue of polyethylene with structural unit –CF2–, demonstrates this change as expected, but its very high melting point makes it difficult to mold.
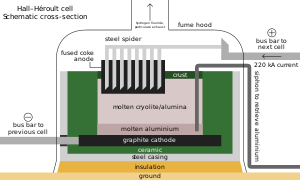
[170] About 20% of manufactured HF is a byproduct of fertilizer production, which produces hexafluorosilicic acid (H2SiF6), which can be degraded to release HF thermally and by hydrolysis: Moissan's method is used to produce industrial quantities of fluorine, via the electrolysis of a potassium bifluoride/hydrogen fluoride mixture: hydrogen ions are reduced at a steel container cathode and fluoride ions are oxidized at a carbon block anode, under 8–12 volts, to generate hydrogen and fluorine gas respectively.
[176] While preparing for a 1986 conference to celebrate the centennial of Moissan's achievement, Karl O. Christe reasoned that chemical fluorine generation should be feasible since some metal fluoride anions have no stable neutral counterparts; their acidification potentially triggers oxidation instead.
He devised a method which evolves fluorine at high yield and atmospheric pressure:[177] Christe later commented that the reactants "had been known for more than 100 years and even Moissan could have come up with this scheme.
[185] Fluorine is monoisotopic, so any mass differences between UF6 molecules are due to the presence of 235U or 238U, enabling uranium enrichment via gaseous diffusion or gas centrifuge.
[5][65] About 6,000 metric tons per year go into producing the inert dielectric SF6 for high-voltage transformers and circuit breakers, eliminating the need for hazardous polychlorinated biphenyls associated with oil-filled devices.
[186] Several fluorine compounds are used in electronics: rhenium and tungsten hexafluoride in chemical vapor deposition, tetrafluoromethane in plasma etching[187][188][189] and nitrogen trifluoride in cleaning equipment.
[65] As with other iron alloys, around 3 kg (6.6 lb) metspar is added to each metric ton of steel; the fluoride ions lower its melting point and viscosity.
[92][196] Halogenated refrigerants, termed Freons in informal contexts,[note 16] are identified by R-numbers that denote the amount of fluorine, chlorine, carbon, and hydrogen present.
[227] Tricyclics and other pre-1980s antidepressants had several side effects due to their non-selective interference with neurotransmitters other than the serotonin target; the fluorinated fluoxetine was selective and one of the first to avoid this problem.
Many current antidepressants receive this same treatment, including the selective serotonin reuptake inhibitors: citalopram, its enantiomer escitalopram, and fluvoxamine and paroxetine.
[237][238] Fluorine-18 is often found in radioactive tracers for positron emission tomography, as its half-life of almost two hours is long enough to allow for its transport from production facilities to imaging centers.
[250] Both the WHO and the Institute of Medicine of the US National Academies publish recommended daily allowance (RDA) and upper tolerated intake of fluorine, which varies with age and gender.
[273] One-fifth of the lethal dose can cause adverse health effects,[274] and chronic excess consumption may lead to skeletal fluorosis, which affects millions in Asia and Africa, and, in children, to reduced intelligence.
[274][275] Ingested fluoride forms hydrofluoric acid in the stomach which is easily absorbed by the intestines, where it crosses cell membranes, binds with calcium and interferes with various enzymes, before urinary excretion.
[279] One regional study examined a year of pre-teen fluoride poisoning reports totaling 87 cases, including one death from ingesting insecticide.
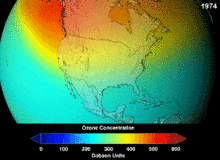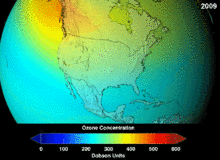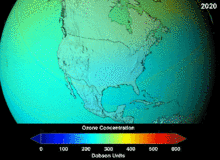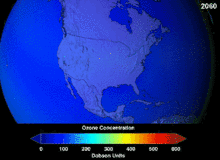
The high stability which suited them to their original applications also meant that they were not decomposing until they reached higher altitudes, where liberated chlorine and bromine atoms attacked ozone molecules.
[291][292][293] PFAAs have been found in trace quantities worldwide from polar bears to humans, with PFOS and PFOA known to reside in breast milk and the blood of newborn babies.
[291][292][295] High doses of PFOS and PFOA cause cancer and death in newborn rodents but human studies have not established an effect at current exposure levels.


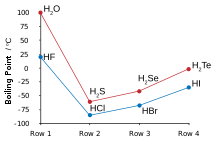
2 molecules that may assume any angle. Other molecules are constrained to planes.










6 current transformers at a Russian railway

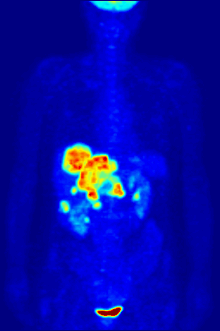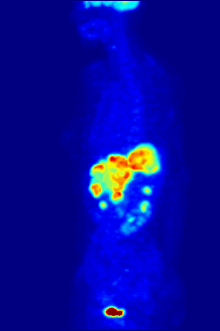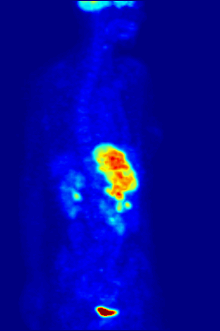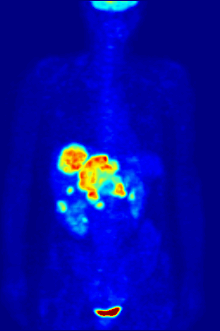
F PET scan with glucose tagged with radioactive fluorine-18. The normal brain and kidneys take up enough glucose to be imaged. A malignant tumor is seen in the upper abdomen. Radioactive fluorine is seen in urine in the bladder.