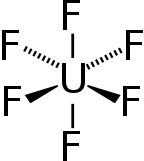
Uranium hexafluoride
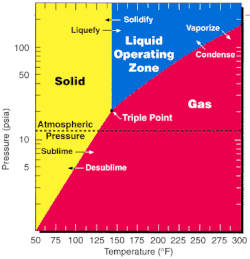
Since the triple point of UF6; 64 °C(147 °F; 337 K) and 152 kPa (22 psi; 1.5 atm);[13] is close to ambient conditions, phase transitions can be achieved with little thermodynamic work.
In addition to its use in enrichment, uranium hexafluoride has been used in an advanced reprocessing method (fluoride volatility), which was developed in the Czech Republic.
In this process, spent nuclear fuel is treated with fluorine gas to transform the oxides or elemental metals into a mixture of fluorides.
Some fission products form nonvolatile fluorides which remain as solids and can then either be prepared for storage as nuclear waste or further processed either by solvation-based methods or electrochemically.
In 2005, 686,500 tonnes of D-UF6 was housed in 57,122 storage cylinders located near Portsmouth, Ohio; Oak Ridge, Tennessee; and Paducah, Kentucky.