Perfluoroether
In general these compounds are structurally analogous to the related hydrocarbon ethers, except for the distinctive properties of fluorocarbons.
The introduction of an ether function to a perfluoro-polymer chain also provides thermoplastic properties to the polymer, making thermal forming possible.
This is a great technological advantage for producing a large variety of shapes (e.g., beakers, funnels, flasks for laboratory uses, etc...) and allows extrusion of highly chemically-resistant tubing.
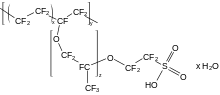
[1] Acyclic perfluoroethers are analogues of diethylether, e.g. O(C2F5)2, such perfluoro(2-ethoxyethane)sulfonic acid (PFEESA).
Methylfluoroalkoxy (MFA) is a polytetrafluoroethylene perfluoro methylvinylether prepared with a different ratio of PTFE and MVE monomers to that used for PFA.